Efficiency
Bionnex Organica products are scientifically substantiated with in vivo studies (Farcoderm)
Study parameters:
- Study title: “Placebo-controlled, clinical-instrumental assessment of the efficacy of cosmetic products for the treatment of alopecia grade II and III and Telogen Effluvium”;
- Study was conducted by Farcodrem (Switzerland, Italy, France).
- In collaboration with the University of Pavia, Professor Fulvio Marzatico, Laboratory of Pharmacobiochemistry, Pharmacology and Toxicology Division
- Study was conducted in June 2011 – July 2012.
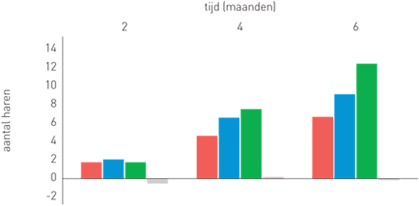
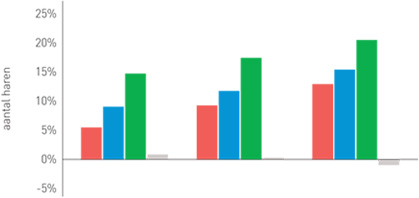
- 120 patients were followed for 6 months.
- Exclusion of pregnant women and women in the phase of breast-feeding.
- Evaluation of the study results 1,2,3,4,5 and 6 months after the start of the treatment.
- 4 groups of 30 persons (men and women between 20 and 55 years old) who suffered from alopecia (grade II and III) or Telogen Effluvium, including 1 placebo group of 30 persons.
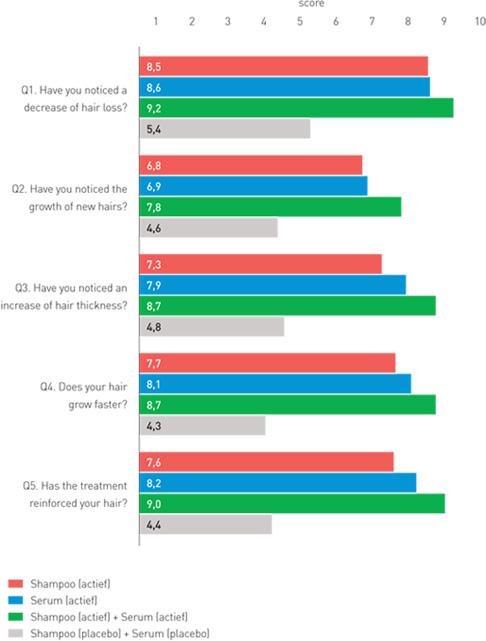
Group 1 (red): Organica anti-hair loss shampoo
Group 2 (blue): Organica anti-hair loss serum concentrate
Group 3 (green): Organica anti-hair loss shampoo and Organica anti-hair loss serum concentrate
Group 4 (grey): Placebo shampoo and placebo concentrate
- Product effectiveness is tested by means of a pull test and a phototrichogram (photographical count of the hairs).
- Before testing, the inclusion of patients was checked by a dermatologist.
Remarkable results: for doctor and patient
| Phototrichogram with the evolution of the number of hairs per group
| Results self-assessment of the patients
|